COVID19 Vaccines Explained
WHICH VACCINE WILL AUSTRALIA USE?
February 2021
Australia’s COVID vaccine rollout scheduled to begin in mid- to late February. Vaccination will commence with workers dealing with international arrivals or quarantine facilities, frontline health workers and those living in aged care or with a disability. Read more: https://theconversation.com/australias-vaccine-rollout-will-now-start-next-month-heres-what-well-need-152612
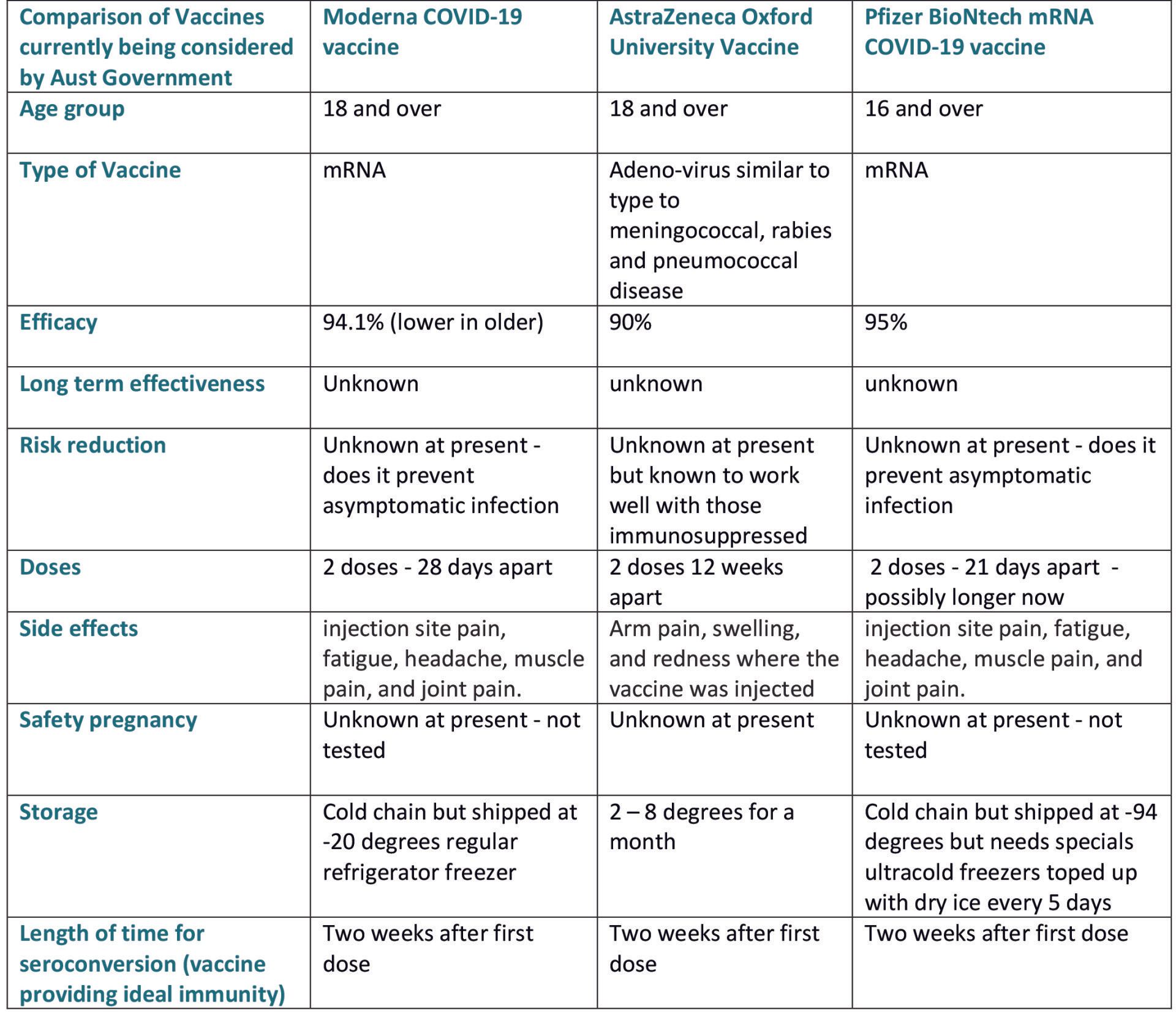
The Australian Government announced on the 6th Jan 2021, that the first rollout of vaccines will be Pfizer-BioNtech of which the Government has secured 10 million doses. Followed by 50 million doses of Oxford AstraZeneca vaccine produced in Victoria. Each of these vaccines will be subject to approval by the TGA (Therapeutic Goods Association).
WHY ARE WE NOT RUSHING TO START VACCINATING IN AUSTRALIA
The vaccines in the UK and the USA have only received ‘emergency approval’ of the vaccines currently being distributed which means stage 3 trials are not finished but the demand is too great to wait as both countries are in dire situations with increasing COVID-19 cases and though we have active cases currently, our situation is much better controlled. The TGA would rather wait to receive the full trial results before giving approval. This is expected to now in February 2021.
In January 2021, there were more than 200 vaccine candidates around the world, 48 of which are in clinical trials. To see the latest on the vaccine landscape click here: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
To ensure Australians will have access to COVID-19 vaccines, the federal government established agreements with suppliers of four of the most promising vaccine candidates. The vaccines have been carefully assessed on advice from a Science and Industry Technical Advisory Group.
So far, the vaccines for which Australia are considering include the AstraZeneca/University of Oxford vaccine, the Novavax vaccine, and the Pfizer/BioNTech vaccine. There is also possibly the Novavax vaccine which is still in phase 3 trials which is manufactured in the USA.
One big question surrounding all of the coronavirus vaccines, is whether they prevent asymptomatic infections as well as illness caused by the virus. Reducing asymptomatic infections, when people can spread the virus without knowing they have it, could have a big impact on slowing the spread of the disease. The Oxford data found some evidence of reduced asymptomatic infections in those who received the half-dose followed by a full dose later.
No one knows for how long coronavirus vaccine provides protection. Some vaccines are effective for years, but for obvious reasons this will take longer to confirm.
Read more: What do we know about the Novavax and Pfizer COVID vaccines
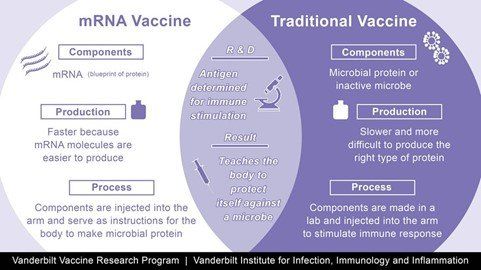
The Pfizer/BioNTech vaccine, which is currently being administered in the UK, is chemically made - using genetic material. These vaccines are created using new technology, known as mRNA, which introduces a genetic code the body can use to make its own viral protein to induce an immune response.
These agreements will only progress in Australia should the vaccines prove safe and effective, as assessed by the TGA, which will look at the quality of the vaccine, the degree of protection it offers, and its safety. At this stage, the TGA have not approved either vaccine to be administered in Australia.
Read More: Information from WHO re vaccine development
Read more: The AstraZeneca Oxford University Vaccine
REACTION - REPORT OF SEVERE ALLERGIES REPORTED FROM THE UK
10th December 2020: Britain's medical regulator has warned that people with a history of serious allergic reactions should not get the COVID-19 vaccine from Pfizer and BioNTech
Key points:
- People with a history of severe allergic reactions to vaccine, medicine or food are now being told to not take the vaccine
- This advice is not uncommon as several vaccines already on the market carry warnings about allergic reactions and subsequent precautionary measures such as the use of ‘epipen’ or having the vaccination is facilities which have resuscitation equipment
- Late-stage vaccine trials in the UK found "no serious safety concerns"
- The medical director of the NHS in England says both affected patients are recovering
WHY KEEP PFIZER/BIONTECH SUPERCOLD?
The most important challenge for development of a mRNA vaccine remains its inherent instability, because it is more likely to break apart above freezing temperatures.
The vaccine needs to be stored at -80 degrees until the last few days, then stored in a vaccine suitable fridge. Dosage is recommended as 2 doses 21 days apart
VACCINATIONS FOR CHILDREN under 12 – UPDATE
The vaccine is being tested in adults 18-54 years of age, 55-85 years of age and adolescents 12-18 years of age. The Pfizer/BioNTech vaccine is the first COVID-19 vaccine to be tested in adolescents.
There is no COVID-19 vaccine being developed specifically for children. So if children are to be vaccinated, they will likely receive the same vaccine as adults.
Children might require a different dosing schedule, but that is not yet clear. The way a child’s immune system reacts to pathogens or vaccines can be different to adults. Age can determine the number of required doses. For example, infants sometimes require more doses of a vaccine than older children. Age can also influence the side-effect profile of a vaccine. For example, mild fever following vaccination can be common in babies and young children.
Vaccine trials are usually done in stages. They typically start with healthy, young and middle-aged adults.
Once a vaccine is confirmed to be safe in these earlier trials, developers then test the vaccine in older and younger age groups.
There are no protocols yet including children in COVID-19 vaccine trials run in Australia. Any Australian studies would only likely examine the immune response and safety in children (phase 1 and 2 trials). They would probably not examine effectiveness (phase 3 trials) because of the low rates of COVID-19 here.
- Researchers will need to examine the dosages, interval between doses, and the number of doses which work best in children.
- This process could take several months
- Kids may not have an approved vaccine until middle or late 2021.
UPDATE RE COVID-19 VACCINE DISTRIBUTION
The ATAGI has provided preliminary advice regarding COVID-19 vaccines strategy for procurement and program delivery for 2021. This document discusses efficacy, duration or protection, targeted groups and modes of delivery. As a summary, those identified as high risk are a priority.
UPDATE AS TO WHO IS PRIORITY TO RECEIVE THESE VACCINES?
The Australian COVID-19 Vaccination Policy, endorsed by the National Cabinet on 13 November 2020 discusses the Federal Government selecting and purchasing the vaccines, decide who gets the vaccines and set the standards for the immunisation program.
State and Territory governments will deliver the immunisation program from specified locations. On page 12 it says: Workplace vaccinations: Some larger corporations and high-risk workplaces may establish workplace vaccination clinics in partnership with State and Territory health services or private providers.
While we are still to see what NSW Health decides and what is the most effective and efficient way for our workforce to receive vaccinations, there is no formal approval from Australia to begin a roll out, as approval must first be sought from the TGA (Therapeutic Goods Administration). It will be interesting to see how the UK responds. Herd immunity isn’t really a consideration for entire population as the BioNTech-Pfizer COVID-19 Vaccine is possible not suitable for children under 12 at this stage. Possible more doses for at risk groups such as those who are immunocompromised. For further information regarding this vaccine, see link from the Australian Government.